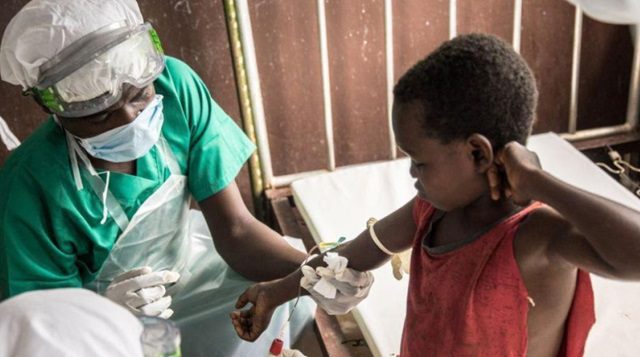
BTN News: The World Health Organization (WHO) has issued a strong call to action, urging for an immediate increase in the production of vaccines against mpox, also known as monkeypox, in response to the rapid spread of a newly emerged, more dangerous strain of the virus. This strain, identified as clade 1, has been causing significant concern due to its heightened contagiousness and severity, particularly in the Democratic Republic of the Congo (DRC) and other African nations. The resurgence of this clade has led the WHO to declare mpox as a Public Health Emergency of International Concern (PHEIC), which is the highest level of alert within the organization, underscoring the urgency and gravity of the situation.
The WHO’s call for increased vaccine production comes at a crucial time when global health experts are scrambling to contain the outbreak. With only a limited number of vaccine doses currently available, the organization is stressing the importance of ramping up manufacturing efforts to meet the rising demand. Margaret Harris, a WHO spokesperson, emphasized the critical need for more vaccines, pointing out that while there are about half a million doses of the MVA-BN vaccine in stock, this is far from sufficient to curb the spread of the virus. The MVA-BN vaccine, produced by Danish pharmaceutical company Bavarian Nordic, is one of two vaccines recommended by WHO experts for combating mpox. The other vaccine, LC16, developed in Japan, is also seen as a vital tool in this fight, though it is currently produced exclusively for the Japanese government and is not commercially available.
The WHO has made a clear appeal to countries that have stockpiled vaccines to donate them to regions experiencing severe mpox outbreaks. This global solidarity is seen as essential in preventing the virus from spreading further and causing more severe public health crises. There is an urgent need for collective action, especially as the virus’s spread is not limited to one region; the risk of it spilling over into other areas is high, making it a global concern.
The history of mpox dates back to 1970 when it was first detected in humans in the DRC, then known as Zaire. It is a zoonotic disease, meaning it primarily spreads from animals to humans, but can also be transmitted through close physical contact with an infected person. Symptoms of mpox include fever, muscle aches, and skin lesions, which can lead to severe complications if not treated promptly. The WHO’s declaration of a public health emergency is aimed at galvanizing international efforts to control the outbreak and prevent it from becoming a more widespread pandemic.
In terms of vaccine availability, the WHO has highlighted that while there is potential to produce more doses quickly, particularly of the MVA-BN vaccine, this will require a firm commitment from buyers. If orders are placed promptly, an additional 2.4 million doses could be made available soon, with a further 10 million doses potentially ready by 2025. However, this is contingent on global demand and the willingness of countries to invest in these vaccines now, rather than waiting until the situation deteriorates further.
For the LC16 vaccine, which remains under the control of the Japanese government, the WHO is actively working with Tokyo to facilitate donations to affected countries. This vaccine, although not commercially available, represents a significant reserve that could be pivotal in the fight against the virus if mobilized effectively.
The WHO’s urgent appeal for increased vaccine production and international cooperation is a reminder of the interconnected nature of global health. The spread of mpox, particularly the more dangerous clade 1, is a stark illustration of how quickly a localized outbreak can escalate into a global threat. It is a call to action for governments, health organizations, and the pharmaceutical industry to work together to ensure that vaccines are produced and distributed equitably, to protect the most vulnerable populations and prevent a larger public health crisis.