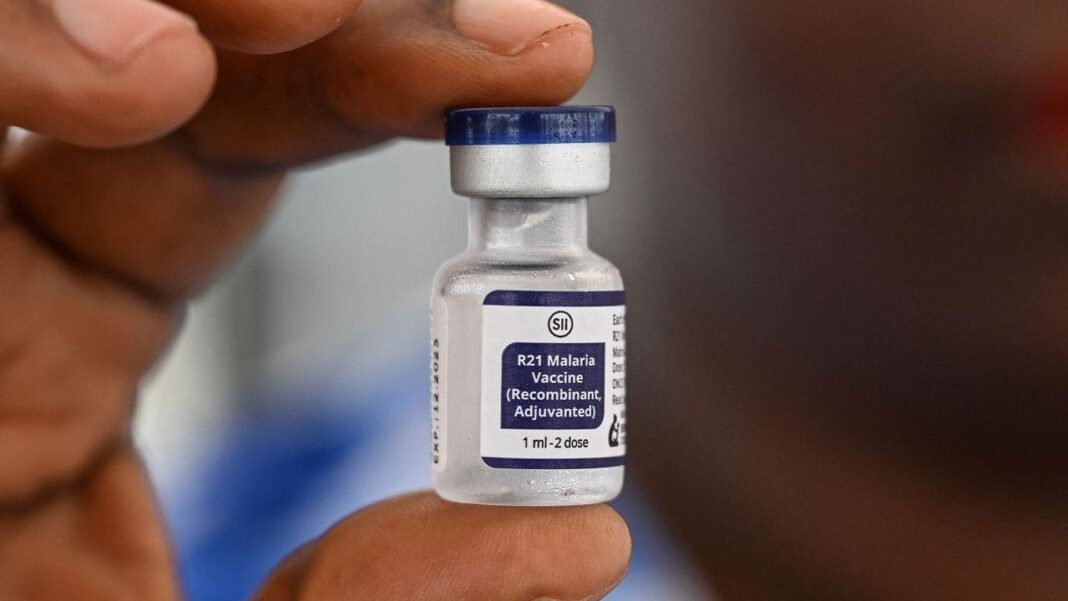
BTN News: In an unprecedented effort to combat malaria—a disease responsible for over 600,000 deaths annually—human volunteers are offering their arms to be bitten by mosquitoes carrying a deadly parasite. These brave individuals are part of a cutting-edge trial aimed at developing a more effective malaria vaccine at Oxford University’s Jenner Institute. By deliberately exposing volunteers to the disease, researchers are advancing what may become a crucial tool in eradicating malaria worldwide. The trial centers around the R21 malaria vaccine, a promising new development that is already generating significant excitement in the scientific community.
The Rise of Human Infection Trials: Speeding Up Vaccine Development
In the fast-paced world of medical research, controlled human infection trials are gaining momentum. These trials allow researchers to test vaccines by intentionally exposing vaccinated individuals to disease-carrying agents like mosquitoes or viruses. While this approach may sound risky, it drastically accelerates the development process for vaccines, potentially saving millions of lives. Volunteers, like those participating in the malaria vaccine trial, help scientists determine whether new vaccines can prevent infection in real-world conditions.
Since 2017, the Jenner Institute has been conducting these experiments, building on over two decades of research into mosquito-borne diseases. The trial participants placed their arms over jars containing mosquitoes infected with malaria parasites, allowing the insects to bite through a mesh covering. The goal? To test if the R21 vaccine could provide sufficient protection to prevent volunteers from developing malaria after exposure.
A Promising Breakthrough: R21 Vaccine Shows 80% Efficacy
The results of these trials have been nothing short of extraordinary. The R21 malaria vaccine has demonstrated an impressive 80% efficacy rate, making it only the second vaccine to gain the World Health Organization’s recommendation for fighting malaria. Countries like Ivory Coast and South Sudan, where malaria takes thousands of lives each year, are now administering the vaccine to vulnerable populations, starting with infants. These breakthroughs underscore how critical human infection challenge studies are in the global fight against infectious diseases.
Ethical Dilemmas in Vaccine Trials: Where Do We Draw the Line?
Despite the progress, the rapid rise of human infection challenge studies has stirred ethical debates. The very nature of these trials—intentionally exposing healthy volunteers to dangerous pathogens—remains controversial. Past abuses, like the infamous Willowbrook and Guatemala syphilis experiments, haunt the medical community, raising concerns about whether modern trials could cross similar ethical boundaries.
According to Adrian Hill, director of the Jenner Institute, advances in medical ethics ensure that today’s trials prioritize the safety and well-being of volunteers. Every precaution is taken to minimize risks, and participants are fully informed of potential dangers before consenting. For instance, volunteers in the malaria trials are exposed to a strain of the parasite that is highly treatable with existing medications. However, for diseases without reliable treatments—like Zika or hepatitis C—the ethical stakes rise considerably.
Why Controlled Infection Trials Are Essential: Time, Lives, and Costs Saved
The need for controlled infection trials becomes more apparent when considering the alternatives. Traditional vaccine trials often take a decade or longer, as they rely on participants coming into contact with diseases naturally in their daily lives. This slow process can be devastating, especially during pandemics or in regions ravaged by diseases like malaria.
Human infection studies cut through this delay by allowing scientists to test vaccines directly, reducing the time and money spent on development. These trials act as early warning systems, identifying potential problems in vaccines before they reach the wider population. In the case of the Dengvaxia vaccine for dengue fever, a controlled trial could have flagged the dangers of administering the vaccine to children who had not been previously infected, potentially averting a public health crisis.
Expanding the Scope: New Diseases on the Horizon for Infection Trials
With the success of vaccines like R21, researchers are expanding the scope of diseases that could benefit from human infection challenge studies. Zika virus, typhoid, cholera, and even hepatitis C are now being considered for similar trials. While some diseases, like Zika, have no current treatment, scientists are carefully weighing the risks against the urgent need for vaccines.
In 2022, a controlled trial exposed 20 women to the Zika virus, closely monitoring their symptoms in a safe, controlled environment. Though results are still pending, this trial could lay the groundwork for future research into mosquito-borne viruses. Some scientists are even discussing the possibility of infection trials for HIV or hepatitis C, though the ethical considerations for such diseases remain highly controversial.
Balancing Risks and Rewards: The Future of Vaccine Research
As the pace of infection challenge studies accelerates, some scientists remain cautious. While these trials offer remarkable benefits, they also carry risks. Eleanor Riley, a professor of infection and immunology, warns that exposing volunteers to diseases with high mortality rates or no treatment options should be done only when absolutely necessary. She emphasizes that not all information gained from these trials justifies the risks, especially when alternative methods could be used.
On the other hand, experts like Arthur Caplan, a bioethics professor, argue that altruism and the desire to advance science justify such trials under the right circumstances. He points to the example of space exploration studies, where volunteers accept risks for the sake of human progress, often with little reward other than the knowledge that they are contributing to the greater good.
Conclusion: Paving the Way for Life-Saving Vaccines
As the world faces an increasing number of infectious diseases, human infection challenge studies may become a critical tool in the fight to save lives. From malaria to Zika, these trials are enabling the rapid development of vaccines that could protect millions. While ethical concerns must be carefully managed, the potential benefits—faster vaccine rollouts, lower development costs, and more lives saved—make this approach an essential part of modern medical research.
In the end, it is the courage and altruism of volunteers—like those in the malaria vaccine trials at the Jenner Institute—that drive this groundbreaking research forward, offering hope for a world free from the devastating impact of infectious diseases.