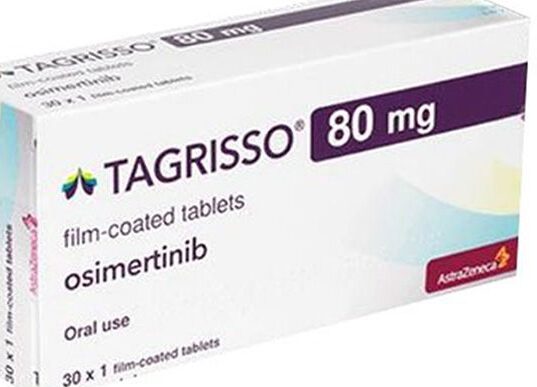
BTN News: The National Institute for Food and Drug Surveillance (Invima) has sounded an urgent alarm over the counterfeit circulation of a cancer medication, Tagrisso (Osimertinib) 80 mg, in Colombia. On September 7, 2024, Invima identified discrepancies in a specific batch, FKHD, with an expiration date of April 2024, and warned the public against its use. The fraudulent drug, typically used for treating non-small cell lung cancer in adults with an abnormal EGFR gene, poses serious health risks due to its unknown contents and lack of safety guarantees.
Fake Cancer Drug Alert: What You Need to Know
Tagrisso, developed by pharmaceutical giant AstraZeneca, is an approved treatment for certain types of lung cancer. However, recent findings by Invima reveal that a counterfeit batch of this drug is being sold illegally in Colombia. The affected batch, FKHD, has distinct packaging inconsistencies that differ from the genuine product. These discrepancies include variations in the thickness of the packaging, incorrect typography, and improper placement of the opening tab on the box.
Spotting the Fake: Packaging Errors Exposed
The counterfeit Tagrisso batch shows multiple errors that can help consumers identify the fake product. Invima has highlighted the following inconsistencies in the packaging:
- Incorrect font and typography: The counterfeit packaging uses a different font and size from the original.
- Box thickness and material: The fake product features a box that is thicker or thinner than the authentic version.
- Incorrect batch code font: The font used for the batch code is different from that of the genuine product.
- Mismatched content: The contents of the counterfeit package do not match the original drug, posing a significant health risk.
Public Safety Risks and Recommendations from Invima
Invima has underscored the serious dangers posed by the counterfeit batch of Tagrisso. Consuming this fraudulent product could have unknown consequences due to its unverified contents and compromised storage and transportation conditions.
To protect public health, Invima recommends the following:
- Avoid purchasing the FKHD batch of Tagrisso (Osimertinib) 80 mg, especially with an expiration date of April 2024.
- Only buy medications from verified sources with a valid health registry.
- Be cautious of online purchases: Many counterfeit drugs are sold on websites, social media, and messaging apps.
Invima’s Call to Action: Reporting and Prevention
Invima urges citizens to report any suspicious or unauthorized sale of Tagrisso or any other medications. Consumers should remain vigilant and prioritize their safety by purchasing medicines only from authorized pharmacies. The agency is working closely with law enforcement to curb the illegal distribution of counterfeit drugs in Colombia.
The Broader Impact of Counterfeit Medications
The spread of counterfeit drugs is a growing concern globally, particularly for life-saving treatments like Tagrisso. Counterfeit medications not only undermine the trust in the healthcare system but also put patients’ lives at significant risk. Health authorities worldwide are increasing their efforts to monitor and prevent the circulation of such dangerous products.
Stay Informed: Protect Yourself from Fraudulent Medications
To stay safe, always verify the authenticity of your medications and stay informed about potential health risks. Visit Invima’s website for the latest updates and detailed guidance on how to identify and avoid counterfeit products.
Final Thoughts
Counterfeit medications like the falsified batch of Tagrisso threaten public health, and it is crucial to heed warnings from regulatory bodies like Invima. By staying informed and vigilant, consumers can help prevent the spread of fraudulent drugs and protect their health.